FDA is Recommending Pausing the Use of Johnson and Johnson Vaccine
FDA is Recommending Pausing the Use of Johnson and Johnson Vaccine in the U.S.
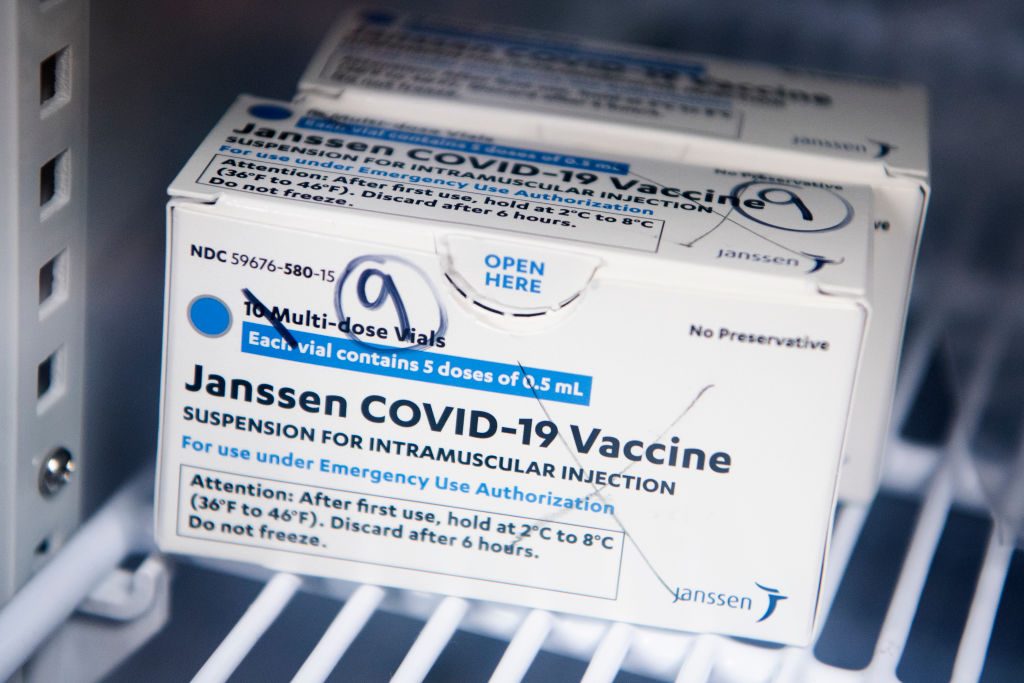
Source: Tom Williams / Getty
The FDA has announced that the distribution of the Johnson & Johnson COVID-19 will be paused after six women in the U.S. had rare blood-clotting after receiving the vaccine. The women were ranging in ages from 18 to 48, and while six people may not seem like a lot precautions are being taken swiftly. There have been approximately 6.8 million shots given with the majority of people not having issues.
The pausing of the Johnson & Johnson vaccine does not affect the other two vaccines from Pfizer and Moderna. But if you did receive the Johnson & Johnson shot and are having to severe stomach pain, leg cramps, or severe headaches you should call your healthcare provider right away. If you do not know what shot you received, check your CDC vaccination card where it is noted what brand shot you received. If you have an appointment scheduled to receive the Johnson & Johnson COVID-19 vaccine, it is advised to call your provider for further directions.
Get Breaking News & Exclusive Contest in Your Inbox:
The Latest:
- Wendy Williams’ Dementia Diagnosis Reversed After New Medical Evaluation
- Paris Jackson reveals drug use caused her to have a perforated septum: “It ruined my life”
- YouTube Could Owe You Money | Techie Tuesday
- From Outkast to Cyndi Lauper, Legends Hit the Stage!
- Veterans Day: 25 Restaurants Offering Free or Discounted Meals in Ohio
- Remember Those Hillman College Days?
- Rumors Are Flying About Diddy in Prison!
- ‘Power Book IV: Force’: Kris D. Lofton & Adrienne Walker Talk Their Characters’ Power, Partnership & Perennial Plotting [Exclusive]
- The Moonwalker, The Myth, The Legend Dazzles In Electric Teaser Trailer For Long-Awaited Biopic ‘Michael’
- Tesla Shareholders Put Elon Musk On Track To Become World’s First Trillionaire



